
Forecasting self-assembly is a job for supercomputers, and the heavyweight programs required can take days or weeks to run. That’s because nature has not been especially forthcoming with her molecular design philosophy. Predicting how molecules self-assemble into 2D sheets is one of the grand challenges of materials science, said Johannes Barth, a physicist at the Technical University of Munich. So many scientists, including Decurtins and his colleagues, want to design materials that assemble themselves. It’s possible to build these 2D materials atom by atom, but doing so is expensive, difficult and time-consuming. And patterns that contain metal ions can be powerful catalysts. These 2D materials can have peculiar and practical properties that depend on how their molecular building blocks are arranged.įor example, it’s possible to arrange molecules into 2D patterns that use electrons as computational bits or to store data.

“It’s just a matter of scale.”ĭecurtins’ patterns weren’t formed by cracks in the earth, but by molecules: they were mosaic-like tilings of molecules in sheets just one molecule thick. Decurtins, a chemist at the University of Bern, was reminded of the materials he had been studying.
#Seesaw molecular geometry example cracked
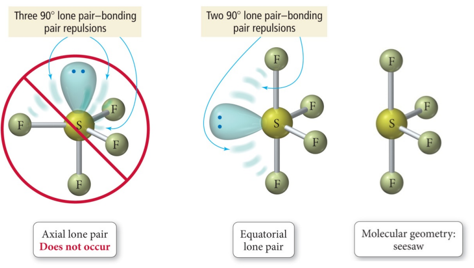
It wasn’t the unusual title that caught his eye, but the pictures on the third page - geological patterns at every scale from cracked permafrost to Earth’s tectonic plates. Consequently, molecules with these geometries always have a nonzero dipole moment.On a Saturday afternoon in the fall of 2021, Silvio Decurtins was leafing through a paper with a title that could have been pulled from a comic book for mathematically inclined teens: “Plato’s Cube and the Natural Geometry of Fragmentation.” Due to the arrangement of the bonds in molecules that have V-shaped, trigonal pyramidal, seesaw, T-shaped, and square pyramidal geometries, the bond dipole moments cannot cancel one another. Consequently, the bond dipole moments cannot cancel one another, and the molecule has a dipole moment. Although a molecule like CHCl 3 is best described as tetrahedral, the atoms bonded to carbon are not identical. In molecular geometries that are highly symmetrical (most notably tetrahedral and square planar, trigonal bipyramidal, and octahedral), individual bond dipole moments completely cancel, and there is no net dipole moment. Other examples of molecules with polar bonds are shown in Figure 2.2.9. Hence the vector sum is not zero, and H2O has a net dipole moment. (b) In H2O, the O–H bond dipoles are also equal in magnitude, but they are oriented at 104.5° to each other. Their vector sum is zero, so CO2 therefore has no net dipole. (a) In CO2, the C–O bond dipoles are equal in magnitude but oriented in opposite directions (at 180°). This charge polarization allows H 2O to hydrogen-bond to other polarized or charged species, including other water molecules.įigure 8 How Individual Bond Dipole Moments Are Added Together to Give an Overall Molecular Dipole Moment for Two Triatomic Molecules with Different Structures. We expect the concentration of negative charge to be on the oxygen, the more electronegative atom, and positive charge on the two hydrogens. Thus a molecule such as H 2O has a net dipole moment. In contrast, the H 2O molecule is not linear (part (b) in Figure 2.2.8) it is bent in three-dimensional space, so the dipole moments do not cancel each other. As a result, the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge. Because the two C–O bond dipoles in CO 2 are equal in magnitude and oriented at 180° to each other, they cancel. Each C–O bond in CO 2 is polar, yet experiments show that the CO 2 molecule has no dipole moment. Such is the case for CO 2, a linear molecule (part (a) in Figure 2.2.8). If the individual bond dipole moments cancel one another, there is no net dipole moment. The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule.

Mathematically, dipole moments are vectors they possess both a magnitude and a direction. In more complex molecules with polar covalent bonds, the three-dimensional geometry and the compound’s symmetry determine whether there is a net dipole moment.
#Seesaw molecular geometry example how to
You previously learned how to calculate the dipole moments of simple diatomic molecules.


 0 kommentar(er)
0 kommentar(er)
