
Mitochondrial outer membrane fusion is almost always coordinated with inner membrane fusion. Although some functional differences between Mfn1 and Mfn2 have been observed, both of these proteins are also able to support mitochondrial fusion by themselves, suggesting that they fulfill partially redundant functions in this process ( Chen et al. Mammals do have two homologs (the Mitofusins Mfn1 and Mfn2), but those are often expressed in the same cells ( Santel and Fuller 2001 Chen et al. Yeast and Caenorhabditis elegans each have only one fuzzy onions or Marf homolog (Fzo1p and FZO-1, respectively) ( Hermann et al. The sequences of Marf and fuzzy onions are similar, but their expression patterns are different so the mutants have different phenotypes: fuzzy onions mutants are sterile whereas Marf mutants are lethal ( Dorn et al. A second mitochondrial outer membrane protein, named Marf, was later shown to mediate fusion in other cell types ( Dorn et al. The first of these was discovered in Drosophila sperm cells, where it was named fuzzy onions for the onion-like and fuzzy appearance of unfused mitochondria in electron micrographs of these mutants ( Hales and Fuller 1997). Drp1, which cycles between the cytosol and the mitochondrial outer membrane, mediates mitochondrial fission.įusion of mitochondrial outer membranes is mediated by a different set of Dynamin family members. Opa1 mediates mitochondrial inner membrane fusion.
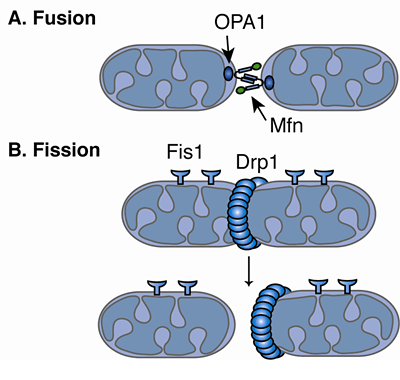
Mitofusins mediate mitochondrial outer membrane fusion in mammals. Mitochondrial fission and fusion are now considered cornerstones for cell survival because of their contributions to health and disease.įunctions of the mitochondrial Dynamin family members. However, in more recent years, the biological relevance of these phenomena has become clear with the discovery of human diseases that are caused by mutations in fission and fusion proteins and the discovery of numerous connections with apoptosis and mitophagy ( Westermann 2010 Chan 2012 Nunnari and Suomalainen 2012 Youle and van der Bliek 2012). Obvious reasons, such as accommodating cell growth, cell division, and the redistribution of mitochondria during differentiation, did not fully explain why mitochondria fuse nor did they explain the high frequencies of these occurrences. The importance of frequent mitochondrial fission and fusion events for cell survival was also not fully appreciated until fairly recently. Because of these morphological changes mitochondria are now known to be very dynamic. In some cells they fuse together, forming a single closed network, whereas in other cells or under different circumstances mitochondria convert into large numbers of small fragments. Mitochondrial morphologies can change dramatically by shifting this balance. Their lengths are determined by the balance between fission and fusion. 1981 Bereiter-Hahn and Voth 1994 Rizzuto et al.
Careful observations, first with phase contrast microscopy, then with vital dyes and finally with targeted fluorescent proteins, showed that mitochondria continually divide and fuse, even in resting cells ( Johnson et al. Renewed appreciation for mitochondrial dynamics emerged some 20 or 30 years ago when technological advances made it much easier to track mitochondria in live cells. For a long time, these observations remained something of a curiosity and they were all but forgotten when electron microscopy popularized the idea that mitochondria exist as isolated sausage-shaped organelles floating in a sea of cytoplasm. Mitochondrial movement and fission were first observed with light microscopy almost 100 years ago ( Lewis and Lewis 1914).


 0 kommentar(er)
0 kommentar(er)
